45 fda requirements food labels
Menu Labeling Requirements | FDA The menu labeling requirements apply to restaurants and similar retail food establishments that are part of a chain with 20 or more locations. In addition, they must be doing business under the... eCFR :: 21 CFR Part 101 -- Food Labeling Smaller formats allowed for Nutrition Facts for certain food labeling under FDA regulation at § 101.9 are not considered to be a size that a ... official of a vending machine operator that voluntarily registers cannot be subject to any State or local nutrition labeling requirements that are not identical to the requirements in 403(q)(5)(H) of ...
PDF A Guide to Federal Food Labeling Requirements for Meat and Poultry Products The food label is important to food companies and consumers alike. A company's most direct (and sometimes only) way to communicate with the consumer is via the food label. For consumers, the food label contains a wealth of information, which allows for informed purchase decisions. The U.S.

Fda requirements food labels
Reliable and Robust Nutrition Food Label Maker Application The U.S. Food and Drug Administration (FDA) is the regulatory body to protect residents in the U.S. from preventable health risks caused by food and zoonotic diseases. FDA rules and regulations enforce nutrition food labeling guidelines, so that residents in the U.S. can enhance and manage their health according to personal requirements. On ... CFR - Code of Federal Regulations Title 21 The information on this page is current as of Jan 06, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.22 Foods; labeling of spices, flavorings, colorings and chemical preservatives. (a) (1) The term artificial flavor or artificial flavoring means any substance, the ... CFR - Code of Federal Regulations Title 21 For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.4 Food; designation of ingredients. (a) (1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be ...
Fda requirements food labels. Guidance for Industry: Food Labeling Guide | FDA It is the responsibility for the food industry to remain current with the legal requirements for food labeling. All new regulations are published in the Federal Register (FR) prior to their... FDA Food Label Compliance - Label Review Fees - fdahelp.us FDA will not review or approve food labels. If the labels are not complying with FDA requirements FDA will consider the product as misbranded and may take regulatory action including detention. LMG's Label review service will help you to confirm your product labels are complying with FDA requirements. we have designed 3 types of label review ... What are the Requirements for a Food Label? - Short Food Labeling Guide Required Food Label Information The FDA requires seven areas of information on food labels for legal sale of these goods. These items include the following information about the food product. All labeling must be in English, though some foreign language is appropriate so long as the English translation is also present FDA Food Packaging Guidelines for 2022 | Newprint Nutritional labels must be printed in all black or one colour type on a white or neutral contrasting background and must be readable. There have been changes in the FDA food labeling requirements regarding nutrition facts, and the transition period has ended on January 1, 2021. Health Canada closely regulates the size and appearance of the NFT.
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and... CFR - Code of Federal Regulations Title 21 For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.9 Nutrition labeling of food. (a) Nutrition information relating to food shall be provided for all products intended for human consumption and offered for sale unless an exemption is provided for the product in paragraph (j) of ... FDA Food Labeling Requirements | Eric F. Greenberg, P.C. From our office in Chicago, Illinois, we represent clients throughout the United States and around the world in a wide array of labeling requirement matters. Contact us to schedule an appointment to speak with a lawyer. You can reach us by phone at 312-977-4647 or via email. Changes are afoot in the world of food contact packaging regulation. Food Labeling | Food and Nutrition Information Center | NAL | USDA FDA's Food Labeling program develops policy and regulations for dietary supplements, nutrition labeling and food standards, infant formula and medical foods. Also conducts scientific evaluation to support such regulations and related policy development. The New and Improved Nutrition Facts Label-Key Changes
FDA Announces Temporary Food Labeling During COVID-19 Pandemic entitled " temporary policy regarding certain food labeling requirements during the covid-19 public health emergency: minor formulation changes and vending machines ," this guidance is one of... FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) Food Product Dating | Food Safety and Inspection Service Federal regulations require a "Use-By" date on the product label of infant formula under inspection of the U.S. Food and Drug Administration (FDA). Consumption by this date ensures the formula contains not less than the quantity of each nutrient as described on the label. The New Nutrition Facts Label | FDA The U.S. Food and Drug Administration (FDA) has updated the Nutrition Facts label on packaged foods and drinks. FDA is requiring changes to the Nutrition Facts label based on updated scientific...
Overview of FDA Labeling Requirements for Restaurants, Similar Retail ... As required by statute, FDA's final rule for nutrition labeling in chain restaurants and similar retail food establishments will provide consumers with clear and consistent nutrition information in...
FDA Food Product Labeling & Packaging Requirements | ESHA Research Mandatory nutrients (total calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, potassium) Placement: In general, place the Nutrition Facts Label on the PDP or the Information Panel, near the ingredient statement. Ingredient Statement
FDA Food Regulations | FDA Food Labeling Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and fish is voluntary. Under FDA's laws and regulations, FDA does not pre-approve labels for food products. FDA regulates dietary supplements under a ...

U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements - Viva FDA - U.S. FDA ...
PDF Food Labeling Guide - FDA Office of Nutrition, Labeling, and Dietary Supplements HFS-800 Center for Food Safety and Applied Nutrition Food and Drug Administration 5100 Paint Branch Parkway College Park, MD 20740 (Tel)...
CFR - Code of Federal Regulations Title 21 Subpart B - Specific Food Labeling Requirements § 101.22 - Foods; labeling of spices, flavorings, colorings and chemical preservatives. § 101.30 - Percentage juice declaration for foods purporting...
A Guide to Federal Food Labeling Requirements for Meat, Poultry, and ... A Guide to Federal Food Labeling Requirements for Meat, Poultry, and Egg Products Guideline ID FSIS-GD-2007-0001 Issue Date August 2007 Full Guideline FSIS-GD-2007-0001 This guidance document assists firms in the development of food labels that meet FSIS requirements. This guidance document relates to FSIS labeling regulations in 9 CFR 317 and 381.

fda labeling requirements for imported food new nutrition lable side by side view enlarged 1 ...
Uniform Compliance Date for Food Labeling Regulations The Food and Drug Administration (FDA or we) is establishing January 1, 2024, as the uniform compliance date for food labeling regulations that are published on or after January 1, 2021, and on or before December 31, 2022. We periodically announce uniform compliance dates for new food labeling requirements to minimize the economic impact of ...
Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow Food Labeling Requirements As Stated By The FDA I. Principal Display Panel 1. Brand Elements 2. Statement of Identity 3. Net Quantity II. Information Panel 1. Ingredient List 2. Instructions to Use 3. Manufacturer Name & Address 4. Country of Origin 5. Product Code III. Nutrient Panel 1. Nutrient Labeling 2. Serving Sizes IV. Claims And Warnings 1.
FDA Labeling Requirements for Food - What You Need to Know Food labeling is required by law for most prepared foods, such as loaves of bread, cereals, canned and frozen foods, desserts, snacks, drinks, etc. Nutrition labeling for raw produce, i.e., fruits and vegetables, and for fish, is voluntary. The FDA refers to these products as "conventional" foods.
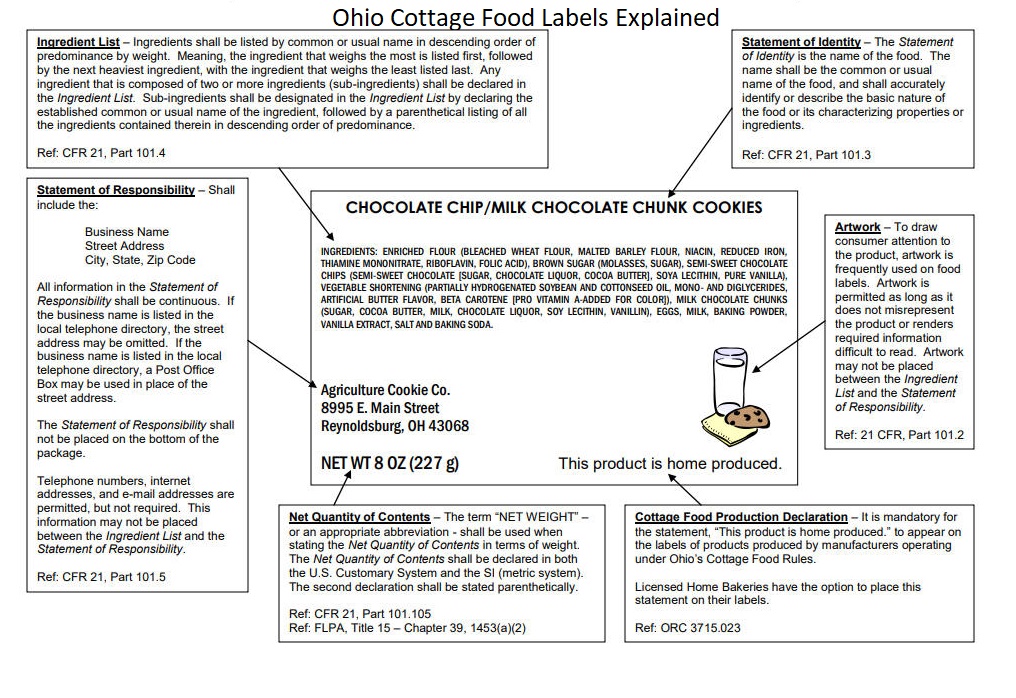
Food Labeling Requirements for FDA Compliant Label Design The FDA requires the following business details on food labels: Business name Street address City or town State Zip Code Indicate if the business name is that of the manufacturer, distributor, importer, etc. If the product is exported or manufactured outside of the US, the country of origin must appear conspicuously on the label for food safety.
Food Labeling Requirements - Register FDA Firms that file for a Small Business Nutrition Labeling Exemption must meet the following requirements: You must employ less than an average of 100 full-time equivalent employees. You must sell less than 100,000 units of that product in the United States in a 12- month period.
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements On May 20th , 2016, the Food and Drug Administration published new FDA labeling regulation for foods, beverages and dietary supplements involving Nutrition Fact table, daily values, serving size, etc. According to the new FDA regulation, labels of food, beverage and dietary supplements must contain Nutrition Facts information that comply with new FDA requirements in respect to the labels ...
CFR - Code of Federal Regulations Title 21 For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.4 Food; designation of ingredients. (a) (1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be ...
CFR - Code of Federal Regulations Title 21 The information on this page is current as of Jan 06, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.22 Foods; labeling of spices, flavorings, colorings and chemical preservatives. (a) (1) The term artificial flavor or artificial flavoring means any substance, the ...
Reliable and Robust Nutrition Food Label Maker Application The U.S. Food and Drug Administration (FDA) is the regulatory body to protect residents in the U.S. from preventable health risks caused by food and zoonotic diseases. FDA rules and regulations enforce nutrition food labeling guidelines, so that residents in the U.S. can enhance and manage their health according to personal requirements. On ...
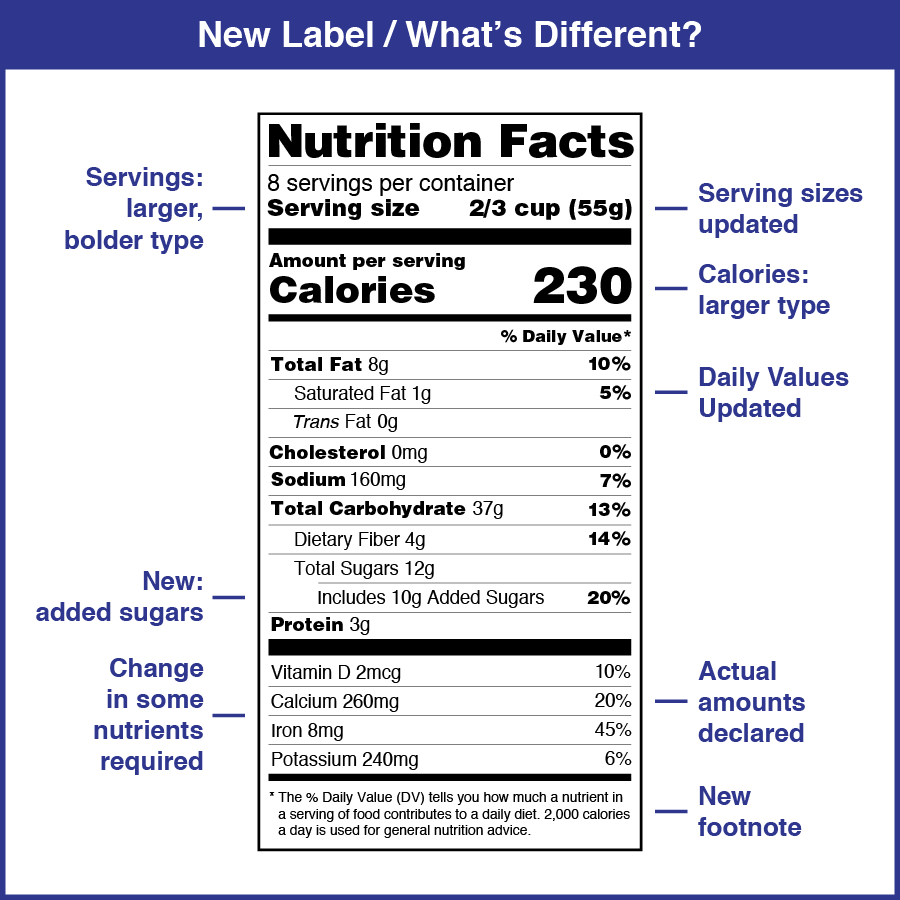





:max_bytes(150000):strip_icc()/__opt__aboutcom__coeus__resources__content_migration__treehugger__images__2014__02__new_vs_old_nutrition_facts_label-aaed1f9fbeee44de91b5bba54f14f9ef.jpg)


Post a Comment for "45 fda requirements food labels"